
Serious Adverse Drug Events (ADEs) reported to the FDA increased all most three times in the last 7 years. Reported deaths increased 2.7 fold, from 5519 in 1998 to 15.107 in 2007. The overall relative increase was 4 times faster than the growth in total US outpatient prescriptions, which grew in the same period from 2.7 billion to 3.8 billion.
What are Serious Adverse Drug Events (ADEs)?
Serious event according to the FDA definition is an adverse event that:
1. resulted in death
2. a birth defect
3. disability
4. hospitalization
5. was life threatening
6. required intervention to prevent harm.
In this research, to prevent double counting, the health outcome was recoded into the following mutually exclusive categories in the following order of priority:
1. death
2. disability
3. all others mentioned above
Reports without serious outcomes were excluded.
Which drugs were included in this survey?
1. prescription drugs
2. biological products except vaccines
3. over-the-counter drugs
Excluded were: medical devices, vaccines, dietary supplements, or illegal drugs. Only the principal suspect drug was used not the secondary or additional drugs used in concomitant therapy.
Method
Data were obtained from excerpts of reports of serious ADEs that were received by the FDA from January 1998 through December 2005. The voluntary reports are submitted either directly to the FDA or to drug companies. The drug companies are required to forward these reports to the FDA if new serious, and unexpected adverse events occur.
More results
- The increase was largely explained by the increase of reports from drug companies of new serious events not on the drug label.
- The proportion of serious events with a death outcome was relatively constant over time: 1998: 15.8% and 2005: 16.8%.
- A disproportionate share of adverse events occurred among elderly not among children younger than 18 years. Even after adjustment for more medication use.
- Among the 15 drugs most frequently named in fatal outcome, 7 were pain killers, 4 had primary effects on the immune system
- The change and overall increase can be atributed to a minority of drugs, 80% of the consequences come from 20% of the causes
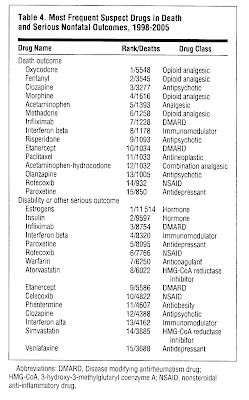
How come?
In the comments the authors suggests some explanations for this nearly 3-fold increase:
- Increasing population and intensive use of drug therapy as measured by prescription volume (25% of the increase)
- a disproportionate contribution of pain meds and drugs that modify the immune system
- 13 prominent new biotechnology products (anti-tumor necrosis factor, interferon alfa and beta) (15% increase)

Limitations of the study
1. It is a collection of voluntary reports not of systematic collected data
2. Only a relationship between drug and effect can be assumed not causality.
3. Reported events also included medication errors, accidental and intentional overdoses, and production problems.
Article discussed:
Arch Intern Med. 2007 Sep 10;167(16):1752-9.
Serious adverse drug events reported to the Food and Drug Administration,
1998-2005.
Moore TJ, Cohen MR, Furberg CD.
PMID: 17846394







4 comments:
Very interesting numbers, not surprising however. Before this study some of the most compelling statistics showing that ADR's were and are a serious problem are as follows.
FDA, Center for Evaluation and Research
-Adverse drug reactions are the fourth leading cause ;
-There are over two million serious Adverse Drug Reactions each year;
-Adverse drug reactions account for approximately 100,000 deaths each year;
Although some ADR events are unavoidable many can be prevented and identified by analyzing available information. The problem is many medical professional don't have the time to do so.
A new consumer health tool DoubleCheckMD makes finding drug-related problems fast and easy. Because DCMD identifies drug-related problems in a fraction of the time it would take other systems, DCMD is helping people recognize drug side effects that would otherwise have been missed for lack of time.
During the recent beta test phase we had a little over 60 visitors and the DoubleCheckmd site pointed out numerous drug-related issues that helped several individuals identify the cause of their symptoms as well as provided life-saving information to 3 individuals.What makes the site unique is that it can do symptom and drug analysis and the interface is incredibly easy to use. For more information on the technology itself visit EnhancedMD Thought this would be a helpful resource for you and your readers!
-Happy Holidays
Well I certainly like the website DoubleCheckMD. Needs some getting used to, but after some time it is very instructive and might indeed help identify drug related problems.
Regards Dr Shock
There is a website that allows you search ADRs by quarter for any suspect drug: www.adverse-drug-reaction.net. There is some useful information about ADRs.
Thanks for the tip, regards Dr Shock
Post a Comment