The information in this review suggests that there is no strong evidence for benefit from using transcranial magnetic stimulation to treat depression, although the small sample sizes do not exclude the possibility of benefit.
This was the conclusion of the authors of the
Cochrane library about Transcranial magnetic stimulation for treating depression.
Since then (2002)
8 randomized controlled trials were published about rTMS and depression
Search strategy in PubMed:"Transcranial Magnetic Stimulation/therapeutic use"[Mesh] AND "Depressive Disorder/therapy"[Mesh] AND ("2003/02/12"[PDat] : "2008/02/10"[PDat] AND English[lang] AND Randomized Controlled Trial[ptyp])
The most recent randomized controlled trial is already discussed on this blog:
At last some good news about rTMS?Considering the outcome on the time point at week 4, Dr Shock is not very impressed by the results. For significant difference with the primary outcome 6 patients had to be excluded from the analysis. The mean difference between active and sham on the severity scales is in the range of 2-3 points, significant but hardly clinical relevant. Absolute figures on response and remission at week 4 are not given in this article. Remission rate at 6 weeks on the HAMD-17 was 15.5% increasing to 22.6% at week 9 with open labeled therapy. Not very impressive.
This same group studied rTMS as form of
long-term maintenance therapy for depression. 10 Patients who had responded to an acute course of rTMS for their depression participated in this open label case series. The maintenance rTMS ranged from 6 months to 6 years for these patients.The maintenance rTMS was started directly after response to the acute treatment. Response was defined as a reduction of 50% or more on the 17-item Hamilton Rating Scale for Depression.
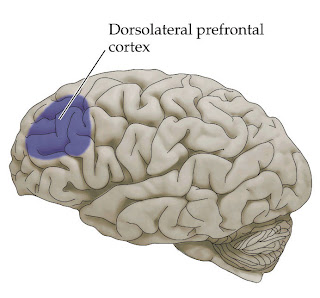
rTMS was applied to the
left prefrontal cortex at 100% of motor threshold. This location is mostly used in depression. The
dosage seems low, usually 120% or 130% above motor threshold is used. The number of pulses per session ranged from 2000-3000. The frequency of sessions was determined by the clinical course of the patient.
 5 Patients
5 Patients experienced
marked benefit of maintenance rTMS (a mean of 257 sessions) with a mean of 2.1 sessions per week. Three of them didn't need additional treatment such as antidepressants.
2 Patients experienced
moderate benefit of maintenance rTMS. Both experienced a recurrence of a depressive episode in a period of 12-18 months. They received a mean of 125 sessions with a frequency of 1.8 per week.
3 Patients received
no benefit of maintenance rTMS.
Two patients were still treated with rTMS as maintenance treatment at the time of publication (2005). No serious side effects were reported.
LimitationsOpen label, no fixed treatment parameters, small number of patients.
In another study
augmentation of amitriptyline with rTMS was studied. 46 Outpatients with non-psychotic depression were randomized to rTMS or sham rTMS (using a placebo coil). During 4 weeks with 5 sessions of stimulation over the dorsolateral prefrontal cortex, 5Hz rTMS, 120% above motor threshold. The subjects received
1250 pulses per day. Seven days prior to the start of rTMS all patients received 50 mg of amitriptyline at evening and the dose was increased by 50 mg every second day. The mean daily dose was 110.2 mg/day which is considered to be very low.
The response rate and remission rates were significantly higher for rTMS compared to sham rTMS, response was 95% versus 46%, which is unusually high. Remission was 54% versus 12%. Real rTMS showed a significant difference with sham rTMS at the end of the first week of this four week trial.
These results are in contrast with 3 earlier add-on studies. Two trials with a sham controlled design couldn't find a significant difference between real rTMS and sham rTMS as add-on strategy. The other trial was an open label trial.
This result is also in strong contrast with a recent add-on study of rTMS to mirtazepine and venlafaxine. This augmentation study is discussed in a recent post on this blog:
rTMS augmentation not useful.Repetitive Transcranial Magnetic Stimulation (rTMS) together with mirtazapine or venlafaxine was not better than these antidepressants with sham rTMS. Response in both treatment groups was 31%, response defined as a reduction of 50% or more on two of three depression severity scales. Even the decrease of scores on the depression rating scales did not differ significantly between real and sham rTMS.There was also no accelerated antidepressant effect, no acceleration of a clinical improvement.
To be continued, stay tuned.......
RUMI, D., GATTAZ, W., RIGONATTI, S., ROSA, M., FREGNI, F., ROSA, M., MANSUR, C., MYCZKOWSKI, M., MORENO, R., MARCOLIN, M. (2005). Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: A double-blind placebo-controlled study.
Biological Psychiatry, 57(2), 162-166. DOI:
10.1016/j.biopsych.2004.10.029O'Reardon, J. (2005). Long-Term Maintenance Therapy for Major Depressive Disorder With rTMS.
Journal of Clinical Psychiatry, 66(12), 1524-1528.
OREARDON, J., SOLVASON, H., JANICAK, P., SAMPSON, S., ISENBERG, K., NAHAS, Z., MCDONALD, W., AVERY, D., FITZGERALD, P., LOO, C. (2007). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial.
Biological Psychiatry, 62(11), 1208-1216. DOI:
10.1016/j.biopsych.2007.01.018